
Further data was collected from the paper medicine chart, the electronic patient management system Health Connect South, the electronic prescribing and administration system MedChart™ and the electronic dispensing system ePharmacy™. This was completed by the nurse retrieving the idarucizumab and included the date and the NHI of the patient.

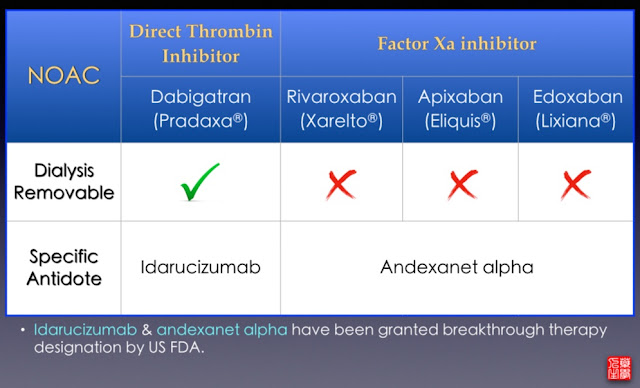
While a 2g IV dose has been shown to be capable of reversing dabigatran in healthy subjects given 220mg orally twice a day of dabigatran, a 5g dose is used.1 Idarucizumab was first registered in New Zealand in December 2015. Idarucizumab binds dabigatran with an affinity 350 times stronger than dabigatran binds thrombin. It irreversibly binds to dabigatran and has a rapid onset and slow offset. It specifically binds protein bound and unbound dabigatran and its active metabolites to form complexes, thus stopping dabigatran’s anticoagulant effects. Idarucizumab is a monoclonal antibody fragment that acts as a reversal agent to the direct thrombin inhibitor dabigatran.


 0 kommentar(er)
0 kommentar(er)
